Because of COVID-19, this year's flu season brings with it heightened patient concerns about contracting the flu. Understandably, hospitals are also concerned as they want to avoid having to treat an influx of patients sick with seasonal flu during the middle of the coronavirus pandemic. To minimize chances of influenza outbreak, the Centers for Disease Control (CDC) encourages everyone over the age of six months to get a flu shot this season.
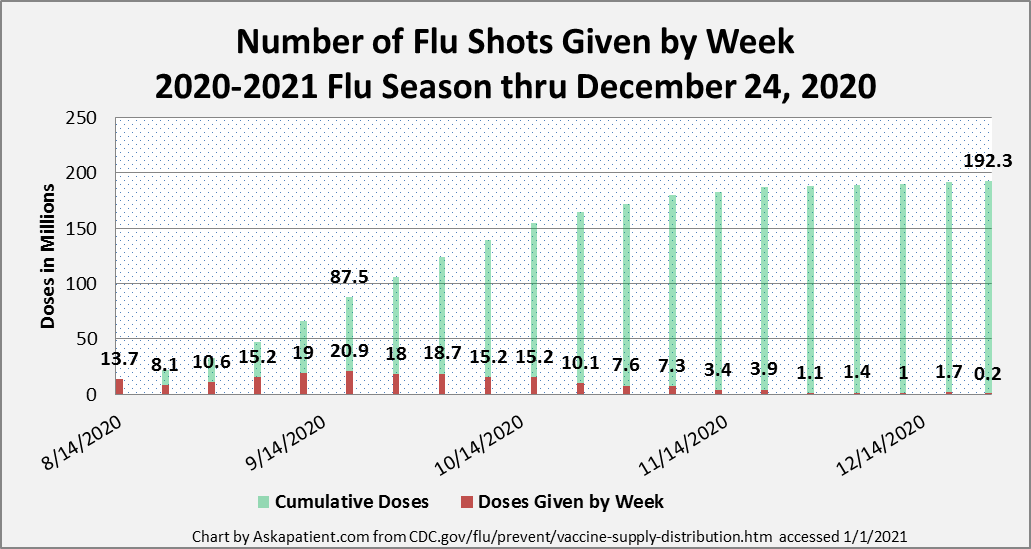
This year, as in previous years, the CDC recommends that people get their flu shots starting in September. While patients may be anxious to get their vaccine right away, an early vaccine might not be effective for the duration of the flu season, which usually lasts from late fall until early spring. The CDC has recommended procedures for safely administering vaccines in various settings. They have also recommended that those with confirmed or suspected COVID-19 wait to receive their flu shot. (See the CDC FAQ referenced below.) The CDC corrected its report that said that as of 2020 November 20, 197.4 million doses of flu vaccine have been administered in the U.S. Instead, as of January 1, 2021, the CDC reports that a total of 192.3 doses of flu vaccine have been provided as of December 24, 2020. The updated chart below presents the weekly number of vaccines given since August. Note that the peak week so far has been the week of September 18, 2020, when 20.9 million flu vaccines were given.
For the entire flu season in 2019-2020,
174.5 million doses were given (see
chart for 2016-2019 flu season comparison).
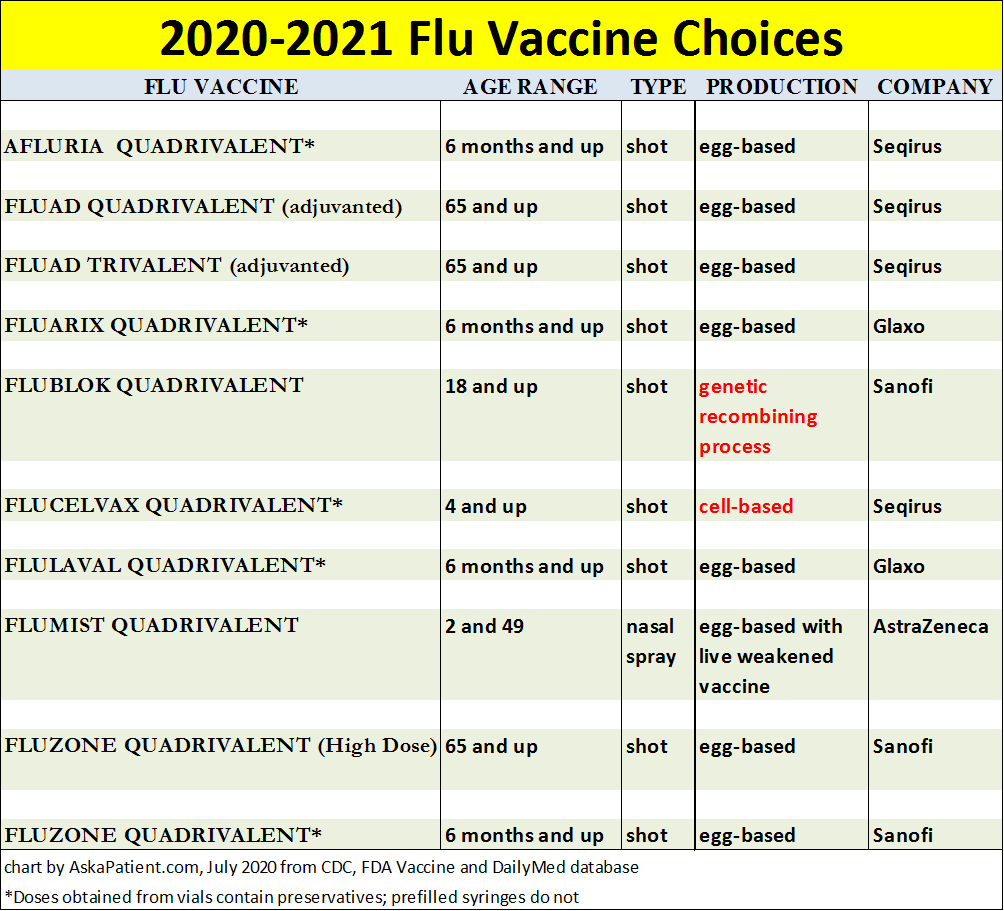
Here is the list of all ten vaccines available this 2020-2021
flu season:
What's different for the 2020-2021 flu season?
With one exception, all flu shots administered this season, including Fluad Quadrivalent and Fluzone High Dose for seniors age 65 and up, will be "quadrivalent," meaning they will contain four influenza virus strains. A limited number of trivalent (three virus strains) version of Fluad for seniors will also be available.
What viruses were selected to be included in the vaccines dispensed in 2020-2021?
Quadrivalent egg-based vaccines will contain and protect against these flu virus strains:
- A/Guangdong-Maonan/SWL1536/2019 (H1N1)pdm09-like virus (updated)
- A/Hong Kong/2671/2019 (H3N2)-like virus (updated)
- B/Washington/02/2019 (B/Victoria lineage)-like virus (updated)
- B/Phuket/3073/2013-like (Yamagata lineage) virus.
Cell-based and recombinant vaccines (highlighted in red in the chart above) will protect against:
- A/Hawaii/70/2019 (H1N1)pdm09-like virus (updated)
- A/Hong Kong/45/2019 (H3N2)-like virus (updated)
- B/Washington/02/2019 (B/Victoria lineage)-like virus (updated)
- B/Phuket/3073/2013-like (Yamagata lineage) virus
For the Fluad trivalent vaccine, these three viruses will be
used:
A(H1N1) pdm09, A(H3N2), and B/Victoria lineage
Special vaccine options available for seniors age 65 and up
For the 2020-2021 flu season, besides regular flu shot options, two shots are again available for seniors who prefer a vaccine that offers extra protection. The CDC does not recommend one of these vaccines for seniors over the other. A recent Duke University (along with CDC, Boston Medical Center, and Cincinnati Children's Medical Center) compared the side effects of Fluzone High Dose with Fluad, and preliminary tables of results suggest that Fluad had lower incidence of side effects, but the quality control review of the study has not yet been completed. (Duke study link)
1. the "high dose" flu shot (Fluzone
Quadrivalent High Dose)
has four times the amount of antigen than is present in a regular vaccine.
Read more about the vaccine on the
Fluzone
High Dose CDC information page.
This vaccine has been
available since 2009.
Some adverse events (which are also
reported after regular flu vaccines) were reported more
frequently after vaccination with Fluzone High-Dose than after
standard-dose inactivated influenza vaccines.
2. the "adjuvanted" flu vaccine (Fluad and
Fluad Quadrivalent) has
an extra ingredient (an oil-in-water emulsion of squalene oil) called MF59,
which helps create a
stronger immune response.
Read more about the vaccine on the
Fluad
CDC information page.
This vaccine was approved in November
2015, and was first available in the U.S. during the 2016-2017
flu season.
Some adverse events (which are also reported after
regular flu vaccines) were reported more frequently after
vaccination with FLUAD. The most common adverse events
experienced during clinical studies were mild to moderate and
were temporary, and included pain, redness at the injection
site, headache, muscle aches, and malaise.
More highlights about this flu season:
- FluMist intranasal
spray is available for patients age 2 - 49
and is the only option this season if you do not want an intra-muscular
shot or the jet injection. The prefilled dosage is .2mL for this vaccine.
FluMist may be hard to find, as only 14 lots so far (as of
October 29) have been approved for distribution by the FDA. (See
FDA chart of vaccine 2020-2021
lot distribution).
- The Jet injector version of
Afluria
is available for this flu season (vaccine label
was updated on 8/27/2020); PharmaJet®
Stratis® Needle-Free Injection System
is available for age 18 through 64 years.
- Special lower-dose version for babies: Afluria Pediatric has half the dosage (.25mL) as the other brands of shots approved for age 6 months to 36 months, which each contain .5mL of vaccine per dose. Children aged 6-35 months should receive 0.25 mL vaccine per dose of Afluria Pediatric, but may require more than one dose at least 4 weeks apart. Fluzone and Flulaval also have been approved for children aged 6 months and up. The Flulaval only comes in .5mL concentration. See vaccine information label for dosing amounts: Fluzone Quadrivalent label and Flulaval label.
- Most of the flu vaccines will be preservative-free in the 2020-2021 season; only those stored in multi-use vials (such as multi-use vials of Fluzone quadrivalent, Afluria quadrivalent, and Flucelvax quadrivalent) contain the preservative thimerosal. Check with your flu shot provider to find out if yours comes in a prefilled syringe (no preservative) or multi-dose vial (will contain a small amount of thimerosal preservative).
- Most people have no or very minor side effects from their flu shot. Some might have the minor effect of a slightly stuffy nose after receiving the live nasally administered vaccine. Other people might have a preference for one type of vaccine (for example an egg-free version) over another. Minor side effects, such as sore arm, might be more severe with high dose or adjuvanted versions compared with a regular flu shot.
How much flu vaccine will be available this season?
Demand for flu vaccines is expected to exceed last year's supply levels, so manufacturers have pledged to boost production by 10%. The CDC reports (as of October 29), that for the 2020-2021 season, manufacturers have projected they will provide as many as 194 to 198 million doses of influenza vaccine for the U.S. market. Flu vaccine supply updates will be provided as they become available at the CDC. Check this page for updates: https://www.cdc.gov/flu/prevent/vaccine-supply-distribution.htm and check this FDA page for quantities distributed by vaccine product name. Over the past four flu seasons, the U.S. has seen a steady increase in vaccination rates. During the 2019-2020 flu season, about 174.5 million flu vaccines were given, about a 3 percent increase over the previous year.
Product availability can vary among different stores, pharmacies, and health care settings, so you will need to check around for your preferred vaccine option.
Not sure if you have COVID-19 or the flu?
The FDA has approved three tests that can differentiate between
the two illnesses:
https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-covid-19-combination-diagnostic-test-ahead-flu
Sources and More Reading:
- CDC's Frequently Asked Questions about this flu season,
including information about the flu viruses and COVID-19 and flu
vaccinations:
https://www.cdc.gov/flu/season/faq-flu-season-2020-2021.htm
-
Data
on how many people receive flu shot. CDC articles provide weekly vaccination data
and projected forecasts for this 2020-2021 flu season.
-
How flu vaccines are made: Article from the CDC explains the 70-year old process
of making vaccines from chicken eggs. Also explains techniques used for newer
cell-based (Flucelvax, approved in 2016) vaccines and recombinant vaccines
(Flublok). Cell-based vaccine
production could alleviate the possibility of shortages in the event of a flu
pandemic, because of quicker production times.
- Information on Recombinant Influenza Vaccine (Flublok)
https://www.cdc.gov/flu/prevent/qa_flublok-vaccine.htm.
Flublok was initially approved in 2013.
- FDA's Lot Release Status of Vaccines page provides
quantities of different vaccines that the FDA has approved for
distribution by manufacturers. As of October 21, a total of 396 lots of
vaccines have been released; of those, 136 are egg-free,
including Flucelvax (70 lots) and Flublok (60 lots).
- More safety information on adjuvanted vaccines:
https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html
- Vaccine Adverse Event Reporting System:
https://vaers.hhs.gov/reportevent.html
-
Health Map Vaccine Finder: Map of Flu Shot
Availability (note: The CDC does not sponsor this service, much of the data has
not been updated for the 2020-2021 flu season as it depends on
individual pharmacies adding their product availability data. But it can be helpful in locating stores or other establishments
that offer flu shots). Updated note: As of April 2021, this site
is NOW sponsored by the CDC and ONLY includes information on
where to find COVID-19 vaccines.
- Information on last year's flu season, 2019-2020:
https://www.askapatient.com/news/flu-vaccines-for-2019-2020.asp
- Related topic: List of Helpful Resources on COVID-19:
https://www.askapatient.com/news/patient-guide-coronavirus-websites.asp
-
Read patient reviews for
Fluzone,
Flucelvax (cell-based),
Afluria, and
Flublok, all quadrivalent vaccines. (Note that reviews with dates from 2018 and 2019 are from the 2018
and 2019 flu seasons.)
-
Related topic: Reviews for Shingles Vaccine:
Shingrix Vaccine Reviews
https://www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2020-2021-season
Although adjuvants make vaccines work better, they can cause
more local reactions (such as redness, swelling, and pain at the
injection site) and more systemic reactions (such as fever,
chills and body aches) than non-adjuvanted vaccines. Also see
the Fluad link above.
VAERS is co-managed by the Centers for Disease Control and Prevention (CDC) and
the U.S. Food and Drug Administration (FDA).
Check this resource list for test directories, where to go for
travel advisories, statistics, and more.
Share your experience with your flu shot by using the List of
2020 Influenza Vaccines with links to rating forms for all 10 vaccines.