The "Open Payments Database" (OPD) is a disclosure program created in 2013 as part of the Affordable Care Act. The program promotes transparency and accountability by helping consumers understand the financial relationships between pharmaceutical/medical device industries and physicians/teaching hospitals. CMS notes that "financial ties between the health care industry and health care providers do not necessarily indicate an improper relationship."
Here's why OPD might be useful to you:
- search the database by physician name to find out if your doctor or surgeon received payments from a pharmaceutical or medical device company, and learn detail about the payment (e.g. if it was for meals, travel, speaking fee, gift, etc.). Many physicians receive the occasional meal delivered by pharmaceutical reps. For example, a physician or group at a practice might be giving up their lunch hour to listen to the rep's presentation. Or, a physician might receive a royalty every time he/she uses a particular device in a procedure.
- If you are a patient at a teaching hospital, search by your hospital name to find out which pharmaceutical or medical device companies made payments to the hospital.
A notable industry trend is increased spending on physicians by biopharmaceutical companies like Genentech, Biogene, and Celgene. Some
pharmaceutical companies, meanwhile, are upping their donations
to patient advocacy groups. Critics say this is an alarming
trend since large corporate donations to the charities might
create conflicts of interest.
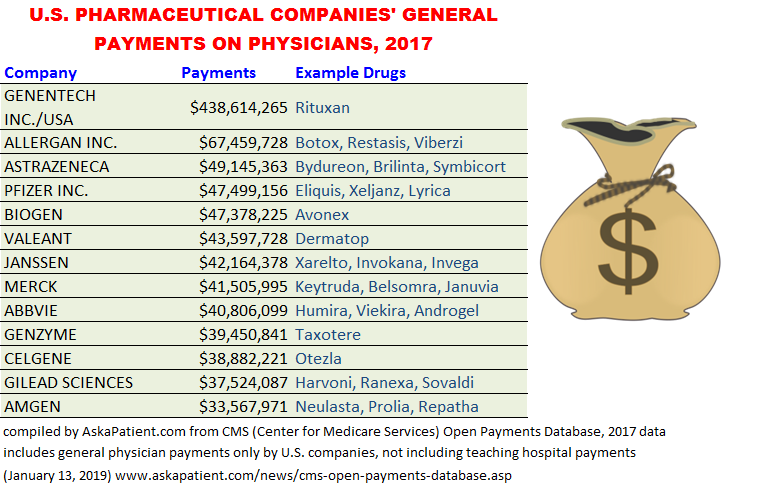
The table below lists examples of companies that spent tens of millions of dollars in 2017 on physicians as reported in the Open Payments Database. Research payments to physicians at teaching hospitals are not included in the totals. Note that California-based cancer drug maker Genentech (including both Genentech Inc. and Genentech USA) spent almost half a billion dollars on general physician payments in 2017. The amounts presented are for U.S. companies only. Links go to patient reviews or ratings forms for drugs.
| PHARMACEUTICAL COMPANIES' PAYMENTS TO PHYSICIANS IN UNITED STATES, 2017* |
||
| Company | Payments | Example Drugs |
| GENENTECH INC./USA | $438,614,265 | Rituxan |
| ALLERGAN INC. | $67,459,728 | Botox, Restasis, Viberzi |
| ASTRAZENECA | $49,145,363 | Bydureon, Brilinta, Symbicort |
| PFIZER INC. | $47,499,156 | Eliquis, Xeljanz, Lyrica |
| BIOGEN | $47,378,225 | Avonex |
| VALEANT | $43,597,728 | Dermatop |
| JANSSEN | $42,164,378 | Xarelto, Invokana, Invega |
| MERCK | $41,505,995 | Keytruda, Belsomra, Januvia |
| ABBVIE | $40,806,099 | Humira, Viekira, Androgel |
| GENZYME | $39,450,841 | Taxotere |
| CELGENE | $38,882,221 | Otezla |
| GILEAD SCIENCES | $37,524,087 | Harvoni, Ranexa, Sovaldi |
| AMGEN | $33,567,971 | Neulasta, Prolia, Repatha |
*As reported in 2018 on the CMS Open Payments Database and Propublica Database; data is subject to change over time.
Sources and More Reading:
- Center for Medicare Services (CMS) Open Payments Database (OPD).
Includes data since August 2016. Search by physician name,
company name (including pharmaceutical and medical device
companies), country of company, and teaching hospital. Results
include number of payments, dollar amounts, drug or product
associated with a payment, and nature of the payment. Includes
data through the end of 2022.
- Additional data analysis is available from
Propublica's
Dollars for Docs Database (covers through December 2018).
- Facts about the Open Payments Data for 2022: https://openpaymentsdata.cms.gov/summary Includes summary information such as:
13.15 million separate general payments were made totaling
$3.70 billion in
2022 (
$3.01 billion in 2018 and $2.82 billion in general payments in 2017).
Also provides research payments amounts and disputed payment amounts.
- OPD in the news: "A potent and controversial new opioid painkiller won federal approval this month based heavily on the work of a University of Minnesota doctor with a history of paid advocacy for the pharmaceutical industry." November 19, 2018. Star Tribune: http://www.startribune.com/u-doctor-in-middle-of-opioid-conflict/500756911/
- Enacted about
2013, the
Physician's Payment Sunshine Act requires that pharmaceutical
and medical device companies disclose gifts to physicians. An
investigation into the policy's impact so far reveals that the
law has not changed physician, consumer, or patient behavior.
The study's authors argue that disclosure is not enough to
address industry relationships in medicine.
https://www.madinamerica.com/2021/04/transparency-not-enough-stem-tide-pharma-influence-medicine/
-"Pharmaceutical Companies' Payments for Physicians"-
Infographic of physician payment table
used in this article